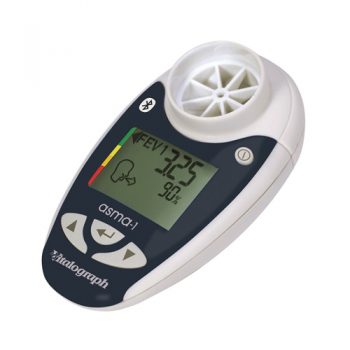
asma-1 bt
$450.00
For the home monitoring of asthma with electronic & hard copy reports
The asma-1™ bt combines all of the features of the asma-1 with the ability to transmit results via Bluetooth using a paired device such as a mobile phone, PDA, PC or home hub. Session data and device ID may be transmitted to Vitalograph Reports, Spirotrac or an API developers’ kit is available for providers of e-Diary, home hub and telemedicine solutions.
- Indicates blow quality, number of blows and diurnal variation
- Device ID automatically transmitted with the data
- Ability to add comments to the report
- Optionally separate graphs of am and pm sessions
- Information presented in tabular and graphical form
- Can automatically name the PDF file
- Subject ID, name, age, height, gender and optional weight field
- Time/date stamp for each session
- Best PEF and FEV1 fields automatically populated
- BMI calculated (from height and weight)
- Personal best FEV1, PEF and zones as set in asma-1
- Trend graphs of FEV1 and PEF
- Reviews (85)
- Specification
Be the first to review “asma-1 bt”
| Product | Vitalograph asma-1 |
| Model Number | 4000 |
| Part Number | 40000, 40400, 40300, 40050 |
| Description | Vitalograph asma-1, Vitalograph asma-1 USB, Vitalograph asma-1 Bluetooth, Vitalograph asma-1 Serial |
| Size | 113 x 63 x 48mm |
| Weight | 55g net |
| Parameters Displayed (Adult version) | PEF & FEV1 |
| Parameters Displayed (Child version) | PEF, FEV0.5, FEV0.75 and FEV1 |
| Operating Temperature Range | 17 – 37°C |
| Measuring Principle | Stator/rotor mass flow meter |
| Scale Intervals | PEF 1L/min FEV1 1L |
| Accuracy | FEV1 better than +/-3% or +/-100ml (whichever is greater) PEF better than +/-5% or +/-25L/min (whichever is greater) |
| Repeatability | Better than 5% or 10L/min, whichever is greater |
| Cleaning | Alcohol wipe, especially the mouthpiece, weekly |
| Flow Range | 0 – 999 L/min |
| Disposal Methods | Recycle |
| Performance standard | ISO 26782:2009; ISO 23747:2007; ATS/ERS 2005 |
| Safety Standards | IEC 60601-1:2005 |
| Designed and manufactured under | CE 0086 ISO 13485:2003 FDA 21CFR820 |












Reviews
There are no reviews yet.